A Sample of Iron Has a Volume of 10.0 Ml
V 1 is the volume to be removed ie aliquoted from the concentrated stock solution. A sample of gas occupies 400.

1 7 Density And Density Problems Chemistry Libretexts
C 2 is the final concentration of the diluted solution.

. Under what pressure would this sample occupy 200. A What is the total concentration of dissolved Fe2 in solution. 49149533 pounds lbs of Iron fit into 1 cubic foot.
Volume in liters at T 35C and P 350 atm. When the volume of the sample is increased to 100 mL and the temperture is increased to 450 degrees celcius the pressure of the gas sample is 0613 atm. What will be the volume of a piece of silver having a mass of 315 g.
Density of iron is equal to 7 873 kgm³. The density of iron is 787 gmL. Suppose that a 20 cm2 sample of iron is immersed in 500 ml of acid solution and the corrosion rate is determined by Tafel extrapolation to be 100 mAcm2.
3 79 A sample of iron occupies a volume of 100 cm3. V 750 mL M 98 g 013. P1V1 P2V2 STP 1 atm 273 K T1 T2.
What is the mass of 45 mL of the metal. A 100 cm3 sample of copper has a mass of 896 g. Which is the correct expression to calculate the mass of the sample using dimensional analysis.
Determine the titration volume required to. 12 A sample of iron has a volume of 100 mL. Which is the correct expression to calculate the mass of the sample using dimensional analysis.
You know that 50 mL have a mass of 925 g so use this as a conversion factor to find. Acetone has a density of 0791 gmL. Pressure in atmospheres at T 42C and V 5 100 L.
F G H J 10 0 787 1. The density of iron is 787 gmL. A 100 cm3 sample of copper has a mass of 896 - 2099461 RWmo4roi3kRollicev RWmo4roi3kRollicev 10302016 Physics.
If a 100 mL sample of unknown liquid has a mass of 7553 g what is the density of the unknown liquid. 07553 gmL dmv d 755310. ML g mL 10 0 787 1.
1492 K from 1219 oC is double of 746 K from 473 oC. What is the volume of a block of iron which weighs 15321 g. If the density of iron is 79 gcm3 what is the mass of the sample.
A 1000-g sample containing an analyte is transferred to a 250-mL volumetric flask and diluted to volume. Density is mass divides by volume so 896g 10cm3 896g cm3 cm3 is a standard unit of volume Advertisement Advertisement New questions in. Assume that the iron corrodes to form Fe2 ions with the evolution of hydrogen.
After a 10 h immersion period. Your goal will thus be to figure out the mass of 1 mL of this metal. Iron has a density of 786 gcm3 1 cm31 mL.
An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. A chunk of iron weighs 432 g. 100 ml ã 787 ml1 g100 ml ã 1 ml787 g100 ml ã 1 g787 ml100 ml ã 787 g1 ml.
Iron has a density of 7874 gcm3. Asample of iron has a volume of 100 ml. 1400 d.
The density of mercury metal is 136 gmL. V metal 155 mL 100 mL 50 mL Now the density of the metal tells you the mass of one unit of volume of this metal. The density of iron is 787 gml.
A sample of iron has a volume of 100 mL. When a 1000 mL aliquot of the resulting solution is diluted to 2500 mL it gives signal of 0235 arbitrary units. Calculate the volume in dL of a piece of iron having a mass of 397 kg.
V 2 is the final volume of the diluted solution. The height of the water rose to 7 ml. What is the volume of gas at 33.
A sample of gas is 220 degrees celcius and 0980 atm occupies a volume of 58 mL. If 1 gallon 37854 liters how many kg should the contents of a 500 gallon container filled with acetone weigh. Iron weighs 7873 gram per cubic centimeter or 7 873 kilogram per cubic meter ie.
A 28 g B 56 g C 14 g D 56 g 25. What is the density of copper. Question Acetone has a density of 0791 gmL.
Titration of the blank gave a titre end-point at 145 mL. Can some one help me with a few chem questions please. 612 d 7 gmL V 200 mL M.
If the mass of the stone was 25 g what was its density. There are 17907 centimoles in 100 grams of Iron. Since youre working with milliliters one unit of volume will be 1 mL.
Draw the following molecule and its ring flipped conformer in. Temperature in degrees Celsius at P 700 atm and V 0973 L. 7 873 kilograms kg of Iron fit into 1 cubic meter.
E If a sample of gas is heated from 473 oC to 1219 oC the volume will increase by a factor of two. ML g mL 13 What mass of nitrogen is required to react with 16 grams of oxygen. Which is the correct expression to calculate the mass of the sample using dimensional analysis.
A second 1000-mL portion of the solution is spiked with 1000 mL of a 100-ppm standard solution of the analyte. A sample of carbon dioxide CO 2 gas has a volume of 152 L at a pressure of 135 atm and a temperature of 33C. This is the volume that results after V 1 from the stock solution has been diluted with diluent to achieve a total diluted volume of V 2.
Determine the following for this gas sample. At 20C 68F or 29315K at standard atmospheric. ML mL g 10 0 1 787.
A sample of helium gas has a volume of 20 L at a pressure of 40 atm. ML mL g 10 0 1 787. A 100 mL sample of supernatant liquid from the system described by equation 1 was titrated with 001022 M EDTA solution to an end-point at 1690 mL.
Note that the density is provided in different units of volume and mass than the desired units of volume. A sample of iron has the same dimensions of 2 cm x 3 cm x 2 cm. ML if the temperature is increased to 819 oC.
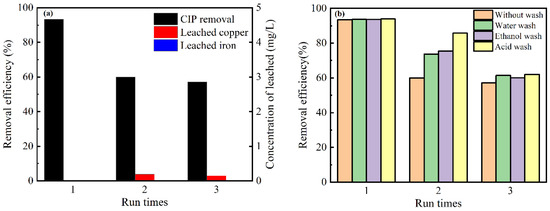
Nanomaterials Free Full Text Adsorption And Fenton Like Degradation Of Ciprofloxacin Using Corncob Biochar Based Magnetic Iron Ndash Copper Bimetallic Nanomaterial In Aqueous Solutions Html

Promoted Adsorptive Removal Of Chromium Vi Ions From Water By A Green Synthesized Hybrid Magnetic Nanocomposite Nfe 3 O 4 Starch Glu Nfe 3 O 4 Ed Rsc Advances Rsc Publishing Doi 10 1039 D1ra00961c

A Piece Of Iron Has A Volume Of 25 Cm 3 And Mass 195 G Find The Density Of Iron In Kg M 3
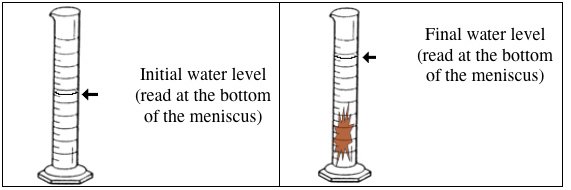
Initially 10 0 Ml Of Water Was Placed In A Graduate Cylinder And Then A Solid Metal Was Immersed In The Water In The Graduated Cylinder At This Point The Volume Of Water
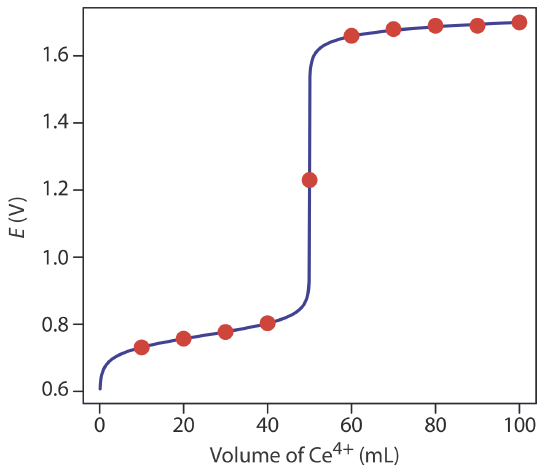
9 4 Redox Titrations Chemistry Libretexts

Ijms Free Full Text New Era In The Treatment Of Iron Deficiency Anaemia Using Trimaltol Iron And Other Lipophilic Iron Chelator Complexes Historical Perspectives Of Discovery And Future Applications Html

Released Test Questions Ppt Video Online Download

Answered 17 The Density Of Copper Is 8 92 G Ml Bartleby

Nanomaterials Free Full Text Adsorption And Fenton Like Degradation Of Ciprofloxacin Using Corncob Biochar Based Magnetic Iron Ndash Copper Bimetallic Nanomaterial In Aqueous Solutions Html

Coatings Free Full Text Thin Protective Coatings On Metals Formed By Organic Corrosion Inhibitors In Neutral Media Html

The Human Innate Immune Protein Calprotectin Induces Iron Starvation Responses In Pseudomonas Aeruginosa Journal Of Biological Chemistry

Evaluation Of The Validity Of A Food Frequency Questionnaire And 24 Hour Dietary Recall To Assess Dietary Iron Intake In Children And Adolescents From The South American Youth Child Cardiovascular And Environmental Study
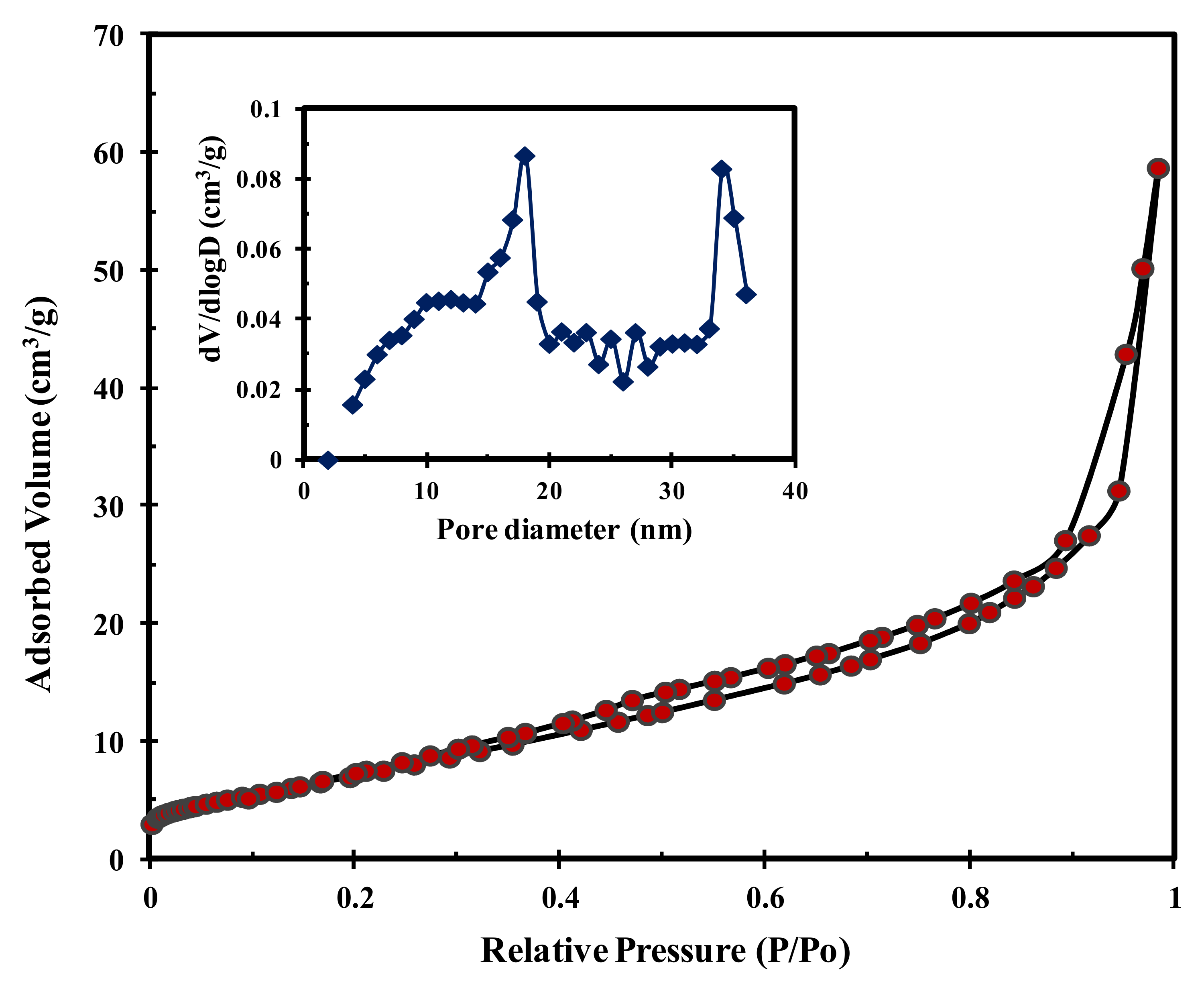
Polymers Free Full Text Biodiesel Synthesis From High Free Fatty Acid Chicken Fat Using A Scrap Tire Derived Solid Acid Catalyst And Koh Html

Solved A Sample Of Iron Ore Weighing 0 2792 G Was Dissolved In An Excess Of A Dilute Acid Solution All The Iron Was First Converted To Fe Ii Ions The Solution Then Required 23 30
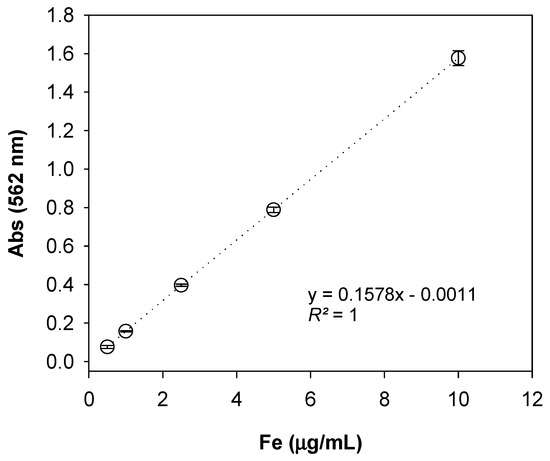
Nutrients Free Full Text Development Of A Paper Based Sensor Compatible With A Mobile Phone For The Detection Of Common Iron Formulas Used In Fortified Foods Within Resource Limited Settings Html

Comments
Post a Comment